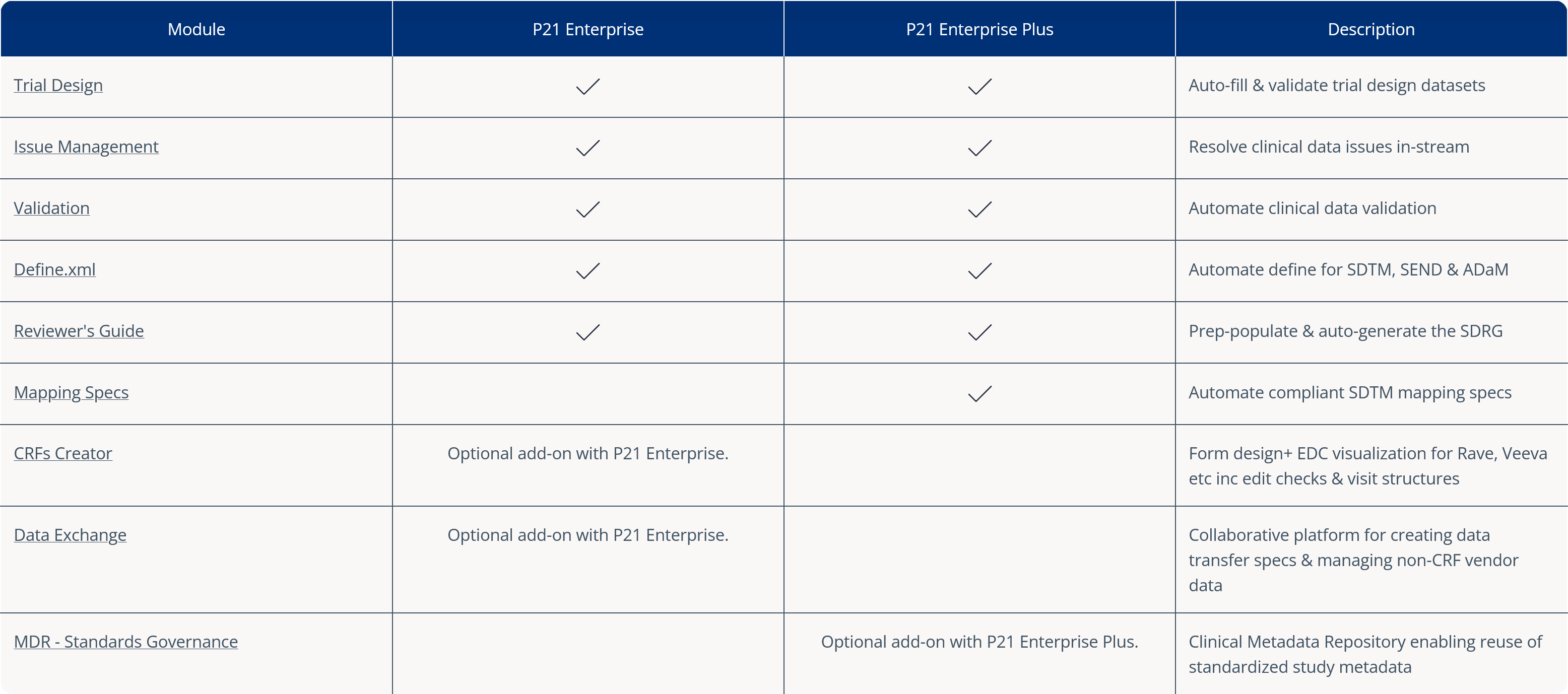
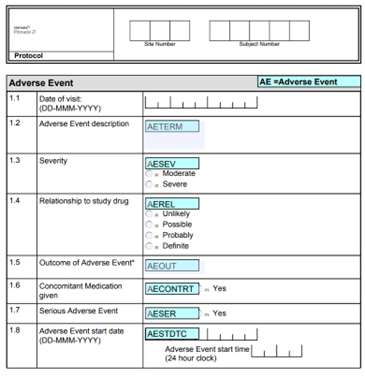
Pinnacle 21® Enterprise CRF Creator allows you to design and build full studies for leading EDC systems, in one central eCRF software platform.
Design forms with question positioning, form dynamics and nested questions. Then preview how eCRFs will look and work for your EDC system in the CRF Creator platform – without needing to build your study. Stakeholder review-edit-approval is done directly in the platform, in real-time, resulting in shorter review cycles, and faster study setup.
Once approved, automatically build the full study file for your EDC with just one click in CRF Creator. Everything you’d expect, from visit structures to EDC specific edit checks is imported into your chosen EDC.