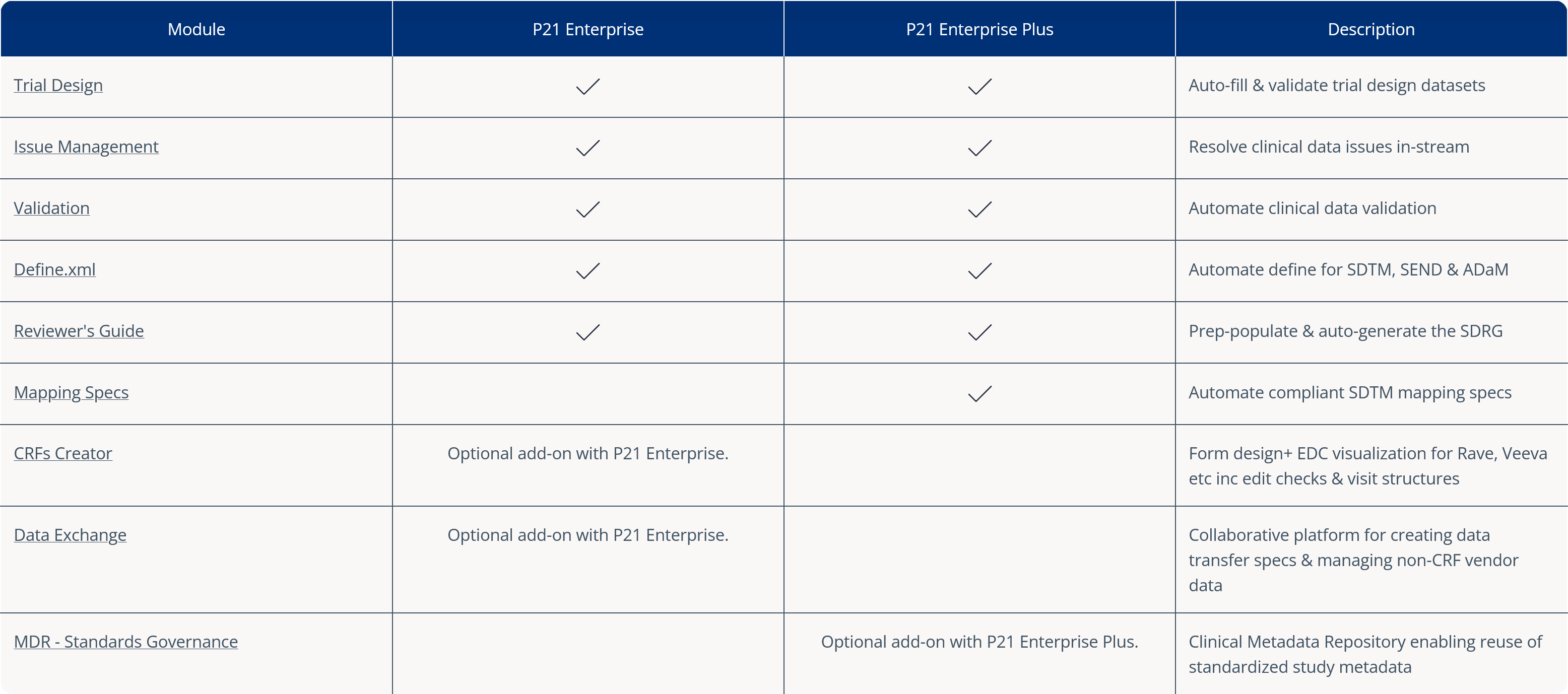
The Pinnacle 21® Clinical Metadata Repository (CMDR) empowers organizations to standardize, reuse, and govern clinical trial metadata with precision. By centralizing metadata management, the CMDR reduces study setup time, enhances data quality, and ensures compliance with CDISC standards.
With Pinnacle 21, study builds are completed in days rather than weeks, enabling faster patient enrolment and earlier trial initiation.