Pinnacle 21®
De-risk submissions and ensure compliance with Pinnacle 21 Enterprise clinical data validation software


为什么要在 Pinnacle 21 Enterprise 中进行临床数据验证?
Pinnacle 21 Enterprise 为临床数据验证提供了一个强大的解决方案,提供无与伦比的监督和控制能力。凭借其先进的仪表板、数据健康度评分和流式验证功能,P21E 确保您的临床试验数据符合监管要求。Our clinical data validation software eases collaboration, enhances data quality, and speeds submission readiness.
预先解决问题
P21E 临床数据验证软件可在提交前识别错误与合规问题。您可依据拒收标准轻松审核数据集,从而提前修复问题。
监测提交就绪状态
通过数据健康度评分,轻松监控提交就绪状态。优先查看需修复的问题,了解其对评分的影响,从而将时间与精力集中在修复关键问题上。甚至可通过预测性评分预判未来结果,助您及早干预存在风险的项目。
掌握全局管控
实时监控提交进度,全面掌握团队活动动态。告别信息孤岛!追踪验证流程前后的变更差异,并利用 20+ 预置报告中的关键绩效指标与深度分析指标,识别跨试验的趋势或不一致性问题。
与监管机构保持一致
P21E’s clinical data validation tool checks for compliance against CDISC standards, controlled terminology, and even dictionaries such as MedDRA and WHODrug. 它还能根据各监管机构的具体规定验证合规性,并提供保持合规的见解和提示。
实时验证
临床数据管理中的持续验证意味着在研究过程中进行数据验证检查,而不是等待提交,因为提交可能会造成延误。Our clinical data validation tool supports SDTM validation rules, ADaM validation rules, Define-XML validation rules, and SEND validation rules.
以您的方式验证
You can upload any organizational standards, terminology, and business rules into our clinical data validation software, to verify CDISC compliance and check for any issues. 这样,就可以根据内部设置对数据进行验证。
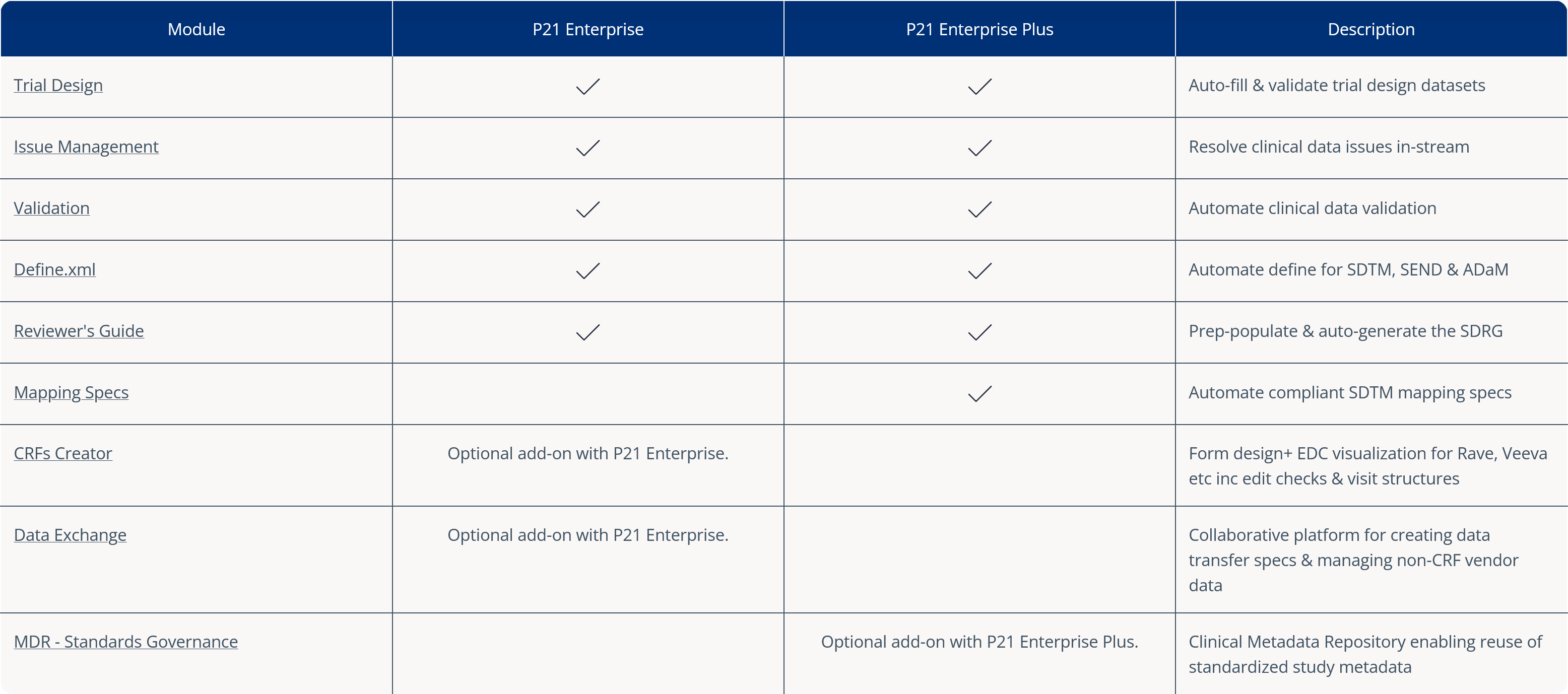
Pinnacle 21 Enterprise Software Suite
With Pinnacle 21 Enterprise as your foundation, add additional functionality according to your requirements. Get in touch to find out more about adding additional modules to your Enterprise platform.
How you’re better off with Pinnacle 21 Enterprise clinical data validation software
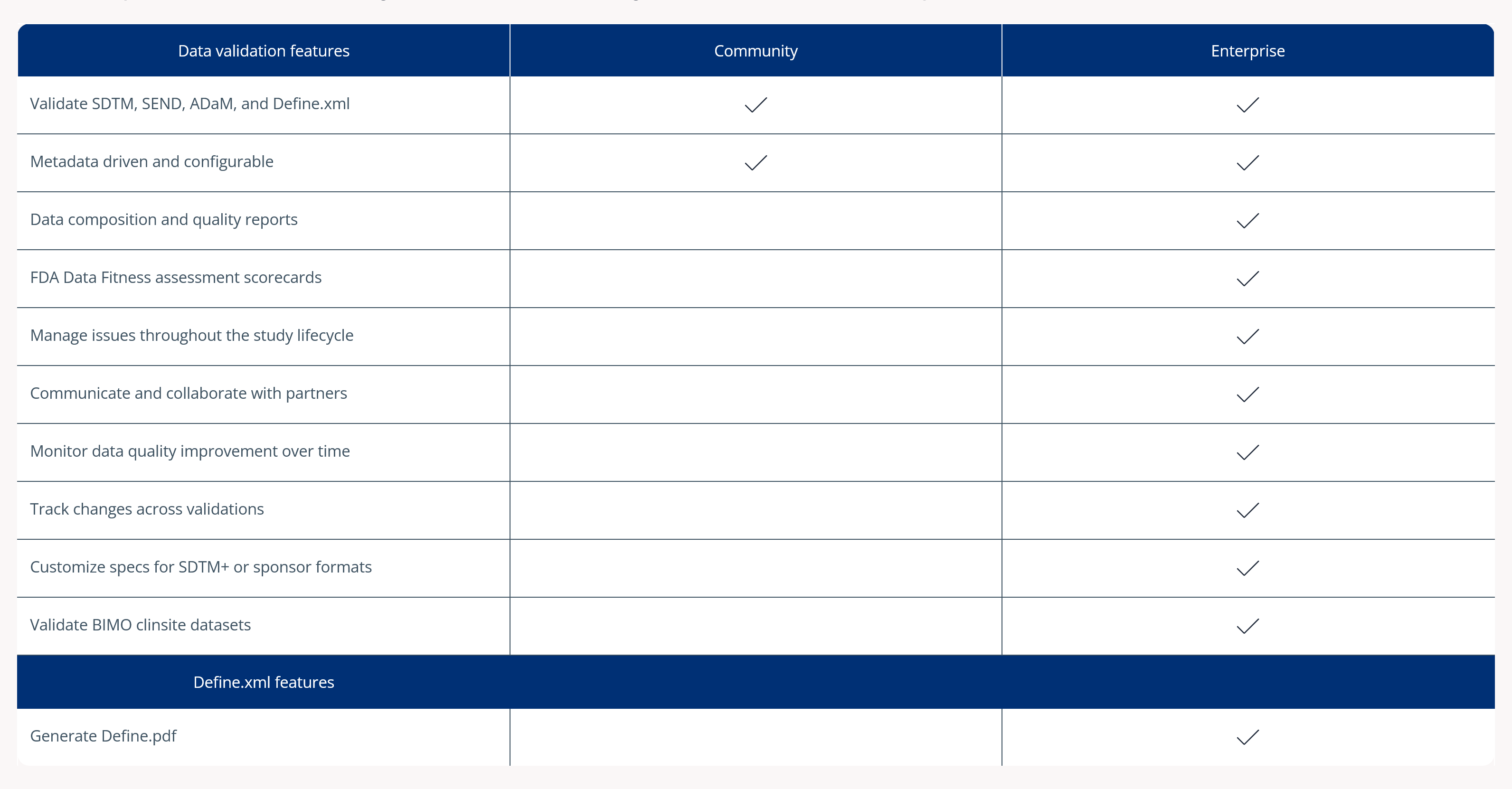
使用 Community?See what you’re missing in Pinnacle 21 Enterprise clinical data validation software
相关产品
为什么选择 Certara?

相关资源
查看全部
With Pinnacle 21, your data is secure
Certara 信息安全管理体系(ISMS)已通过 ISO 27001 认证。我们已实施严密的安全控制措施,完成严格的风险评估,并持续改进。Pinnacle 21 ensures full compliance with global data protection standards, offering peace of mind for sensitive analysis.
预约免费演示
Discover how Pinnacle 21 Enterprise clinical data validation software can improve your end-to-end compliance, and save time. 立即预约演示,亲睹平台运作,了解其如何助力成功提交。
预约免费演示
Make an inquiry about Pinnacle products
Not ready for a demo?
Fill out this form to make an inquiry about the Pinnacle 21 product portfolio, or to discuss your requirements with our team, and we’ll get back to you right away.
常见问题解答
什么是临床数据管理中的数据验证?
临床数据验证是对临床试验数据进行核实的过程,以确保提交高质量、准确的临床试验数据。从本质上讲,数据验证包括确保数据的完整性、一致性和准确性。
如何对临床数据进行验证?
临床数据验证因不同因素而异,如收集的数据类型和所需遵守的监管要求。典型的数据验证流程包括根据一开始定义的规则和标准检查数据。作为验证过程的一部分,将找出错误、不一致或差距,并确定相应的解决方案。
什么是临床数据验证规则?
临床数据验证规则是一组确保数据正确性、完整性与一致性需满足的标准。这些规则规定了数据必须符合的可接受值、格式、关联关系及条件要求。