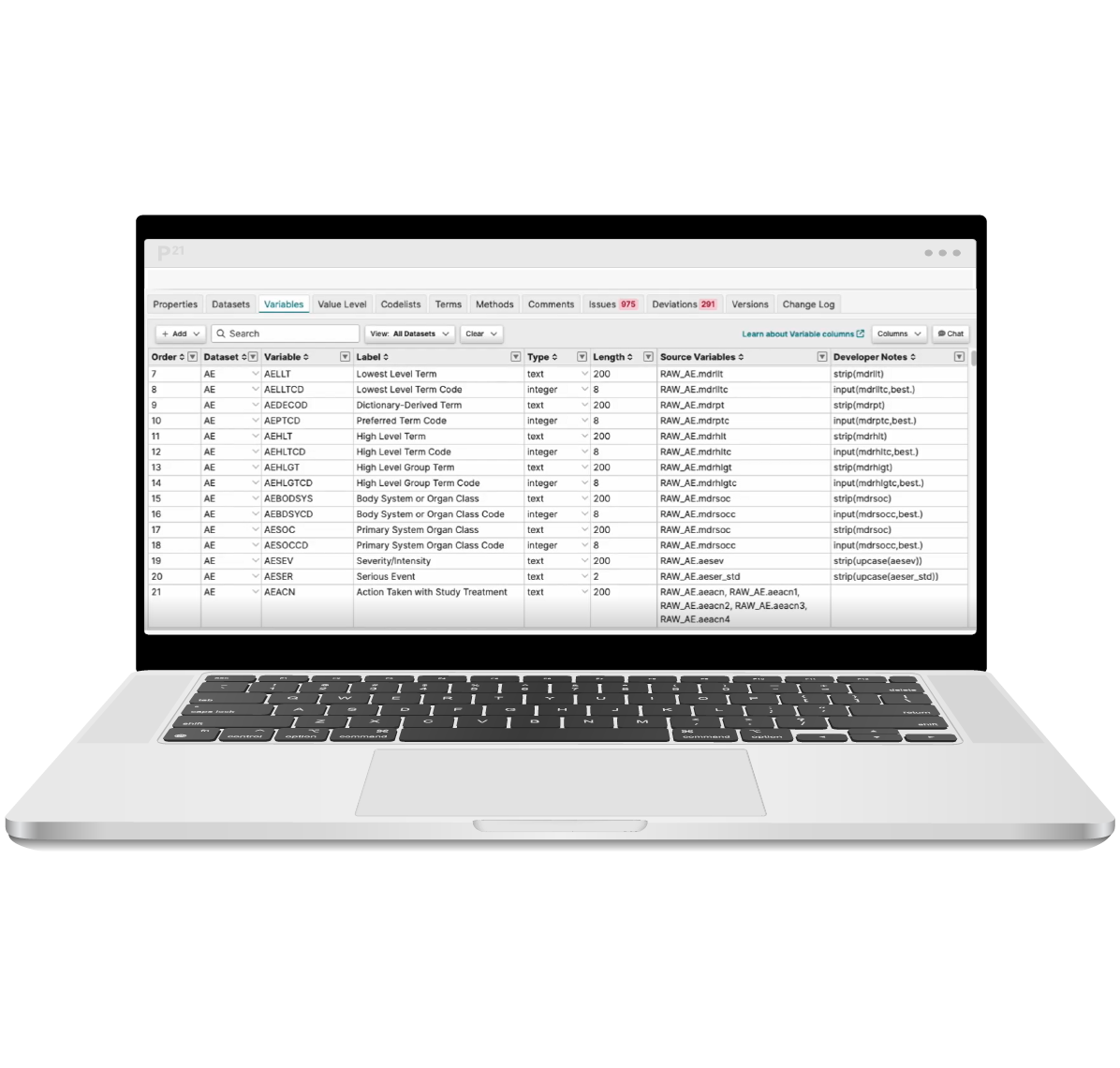
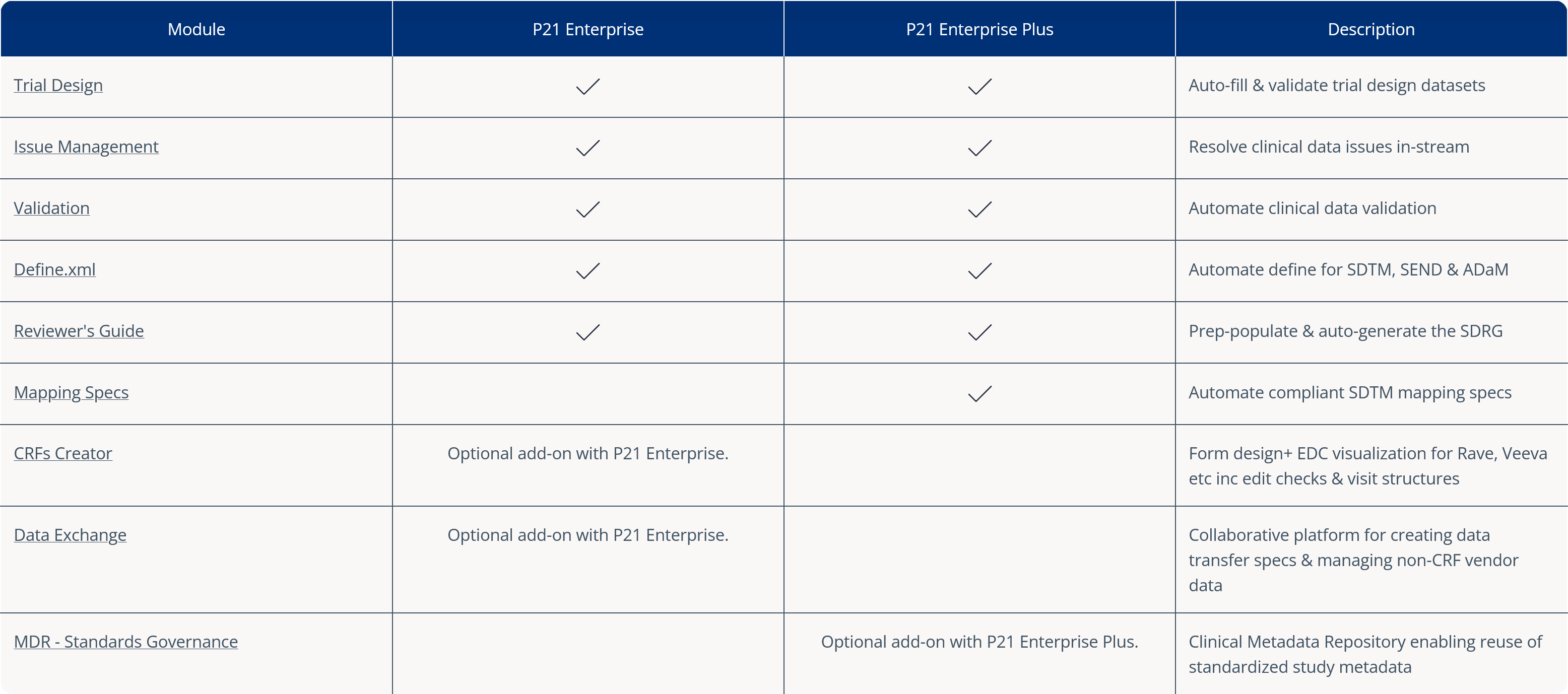
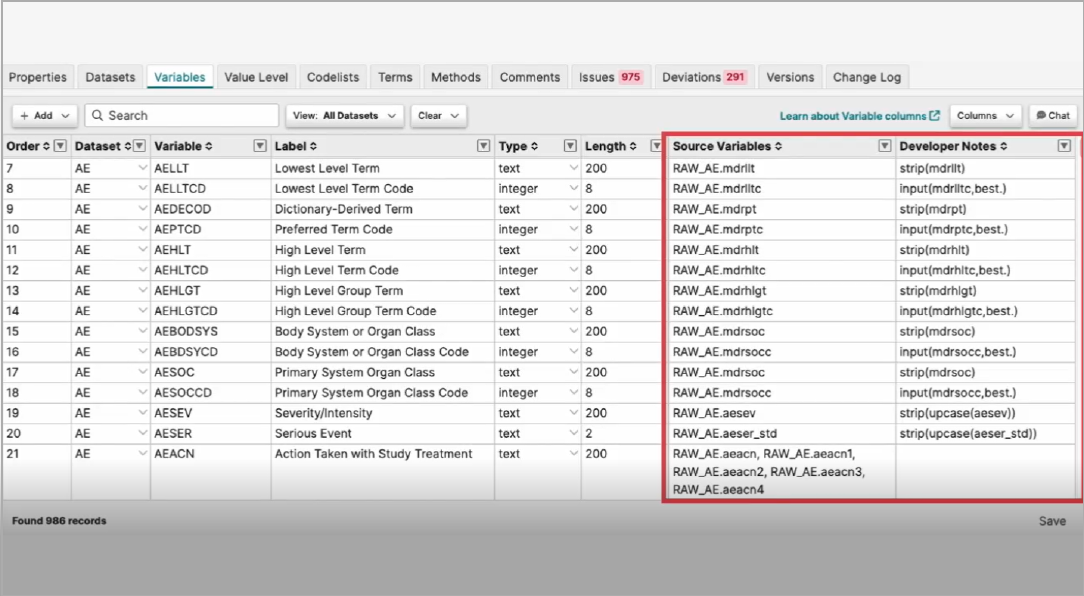
Pinnacle 21’s SDTM mapping tool helps you quickly and easily map source variables to target SDTM formats – avoiding complex, time consuming and error prone manual processes.
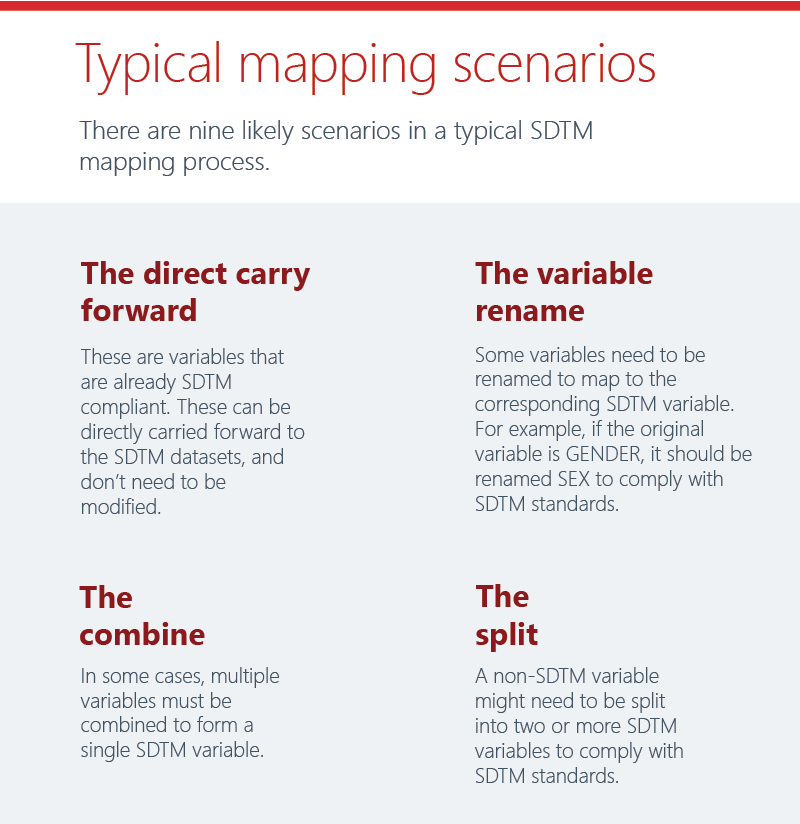
Reuse existing mapping specs from a previous study or established standard to reduce manual rework. Then perform any required re-mappings as identified in the platform. Our mapping algorithm helps with any complex mappings. And errors are identified in-stream, enabling upfront issue resolution.
Now you can produce human-readable or machine-readable SDTM mappings with increased efficiency and compliance!