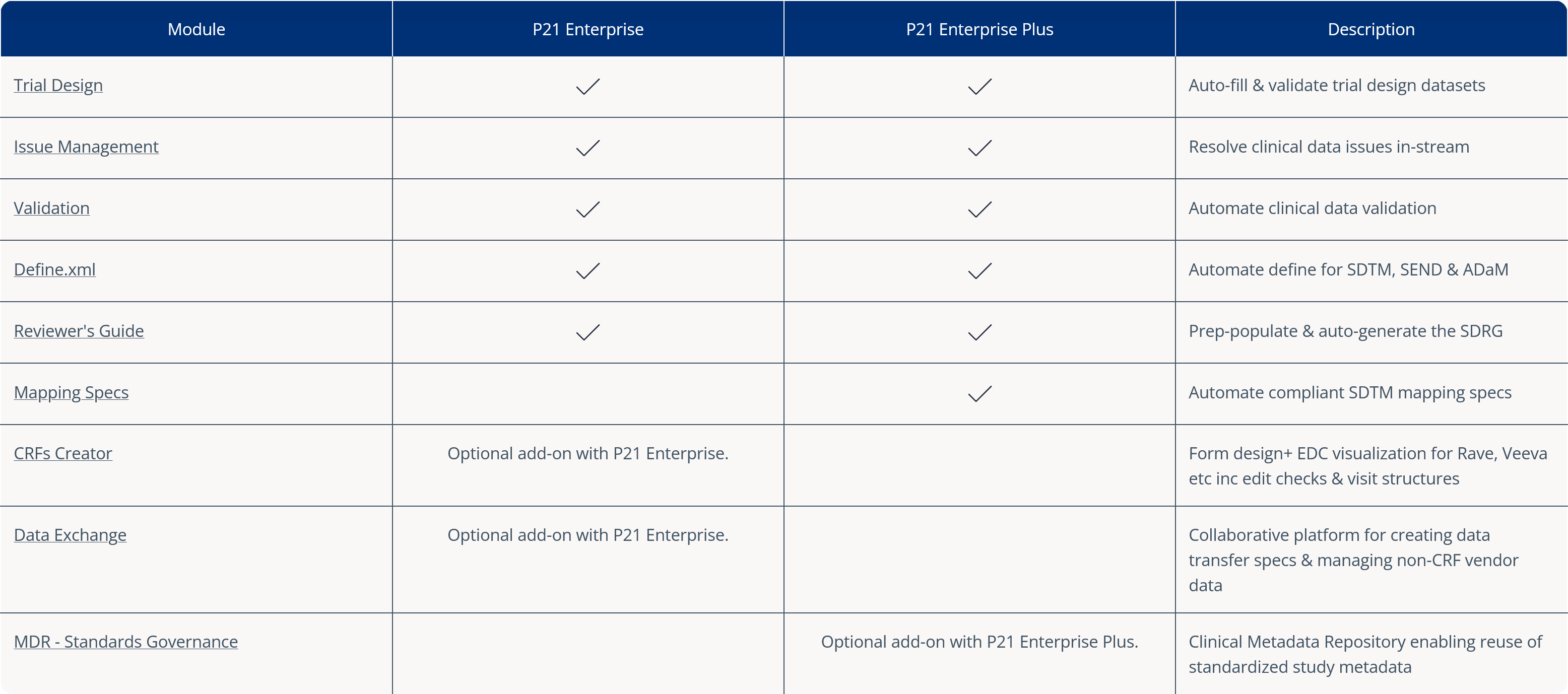
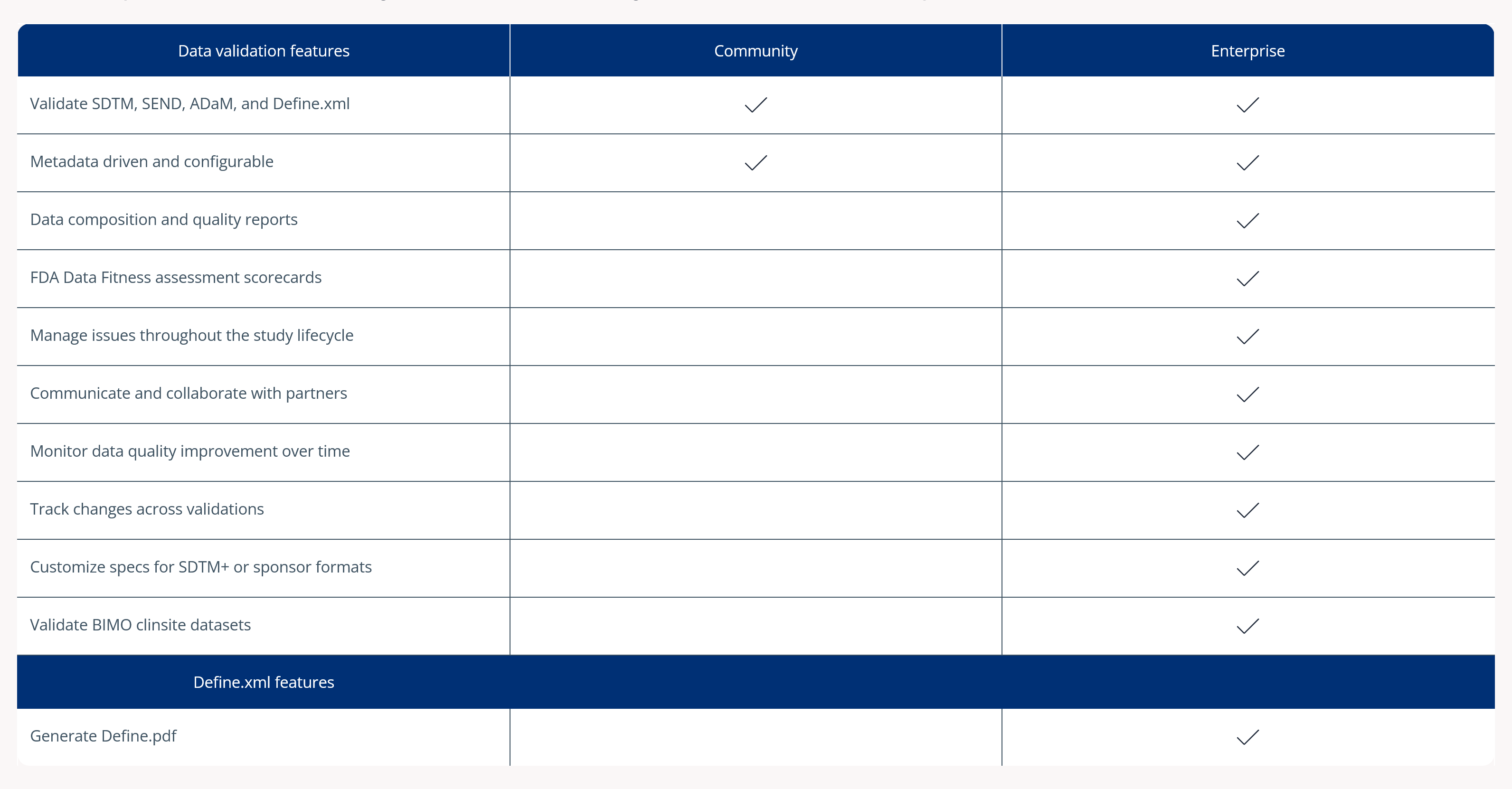
Managing clinical data issues across decentralized teams can be a daunting and time-consuming task. Pinnacle 21 Enterprise (P21E) clinical data issue management software revolutionizes this process by providing a single, collaborative platform for issue resolution.
With P21E, you can assess, triage, and resolve clinical data issues efficiently, while maintaining a clear audit trail for compliance. The platform ensures you always work with the most up-to-date information, helping you track submission readiness in real-time.