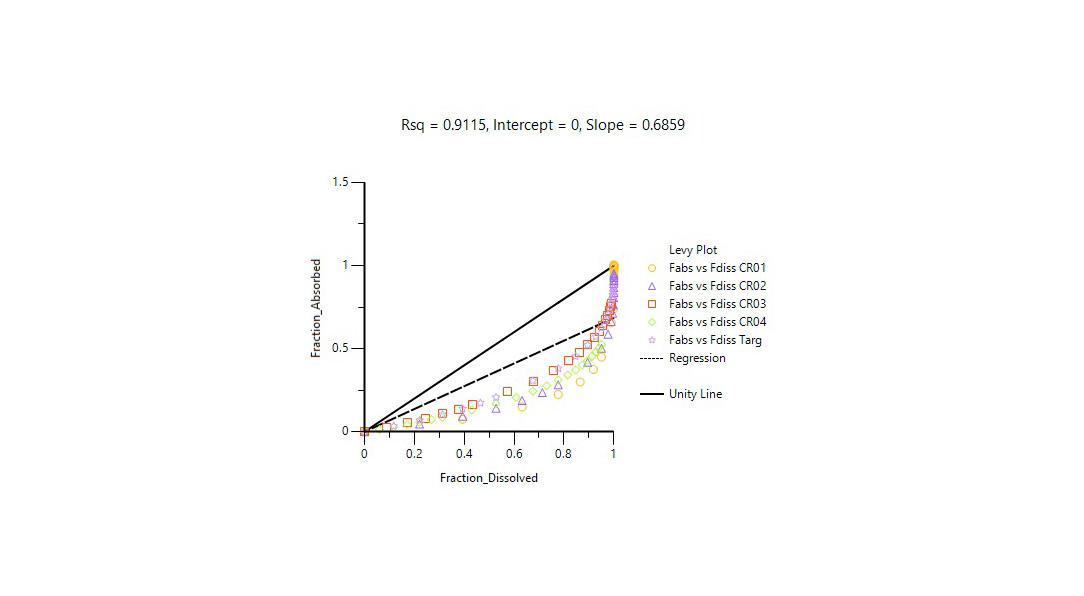
更改药物制剂或生产场地可能成本高昂且耗时。监管机构接受体外-体内相关性(IVIVC)研究,以支持 SUPAC 变更的生物等效性豁免、免除低规格制剂的体内生物等效性研究要求,并满足其他 BE 相关法规要求。
Certara’s Phoenix® IVIVC Toolkit is a leading IVIVC software that provides advanced tools for IVIVC studies, helping scientists improve BE study success with fewer assumptions and better correlation between in vivo and dissolution profiles.