Access to safe and effective medications is critical for pediatric patients, yet 60-70% of medicines prescribed to children are off-label. In Japan, only 30% of new drugs are approved for pediatric use. Unlike the US and the EU, Japan has no regulation mandating pediatric drug development. Still, Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) has been working to promote pediatric drug development for the nation’s children.
Pharmaceuticals and Medical Devices Agency
The PMDA’s purpose is to ensure the timely and appropriate review of new drug applications and to promote developing innovative and effective pharmaceuticals and medical devices. It partners with pharmaceutical companies seeking to develop and market new products in the Japanese market.
PMDA Pediatric Drugs Working Group
The PMDA Pediatric Drugs Working Group (WG) is a project team within the agency. It reviews and assesses pediatric drug development plans and clinical trial data submitted by pharmaceutical companies to ensure that they meet regulatory requirements and ethical standards.
Current pediatric regulations in Japan
Japan has several frameworks in place to provide guidance on ethical and scientific considerations related to pediatric clinical trials:
- Post-marketing safety and efficacy for new drugs are reviewed for a certain period after the approval for marketing authorization. Extension of this re-examination period provides time for pharmaceutical companies to develop pediatric formulations for drugs already approved for adults.
| Re-examination period | Drug type |
|---|---|
| 10 years | Orphan Drugs, Drugs that need to be surveyed by pharmacoepidemiological method |
| 8 years | Drugs with new active ingredients |
| 4 years | New combination drugs, Drugs with a new route of administration |
| 4 – 6 years | Drugs with new indications, Drugs with a new dosage |
- The PMDA has also issued guidelines on the clinical evaluation of drugs in pediatric patients. For example, the guidelines recommend that pediatric patients aged 10-12 years and older can be evaluated with adults.
- The drug reimbursement premium system provides additional reimbursement to drugs approved for pediatric use. This system encourages pharmaceutical companies to invest in pediatric drug development.
- The Council for Unapproved and Off-Labeled Drugs with High Medical Needs, established by the Japanese Ministry of Health, Labour and Welfare (MHLW), offers early access to unapproved and off-label drugs for patients with serious and life-threatening diseases, including pediatric patients. The council identifies highly-needed unapproved drugs/indications, requests pharmaceutical industries to develop or submit a new drug application (NDA) for designated drugs/indications and fast-tracks the review process by accepting public knowledge as the basis for approval.
- The Pediatric Clinical Trial Network is a collaboration between the Japanese government and academic institutions that supports conducting pediatric clinical trials in Japan.
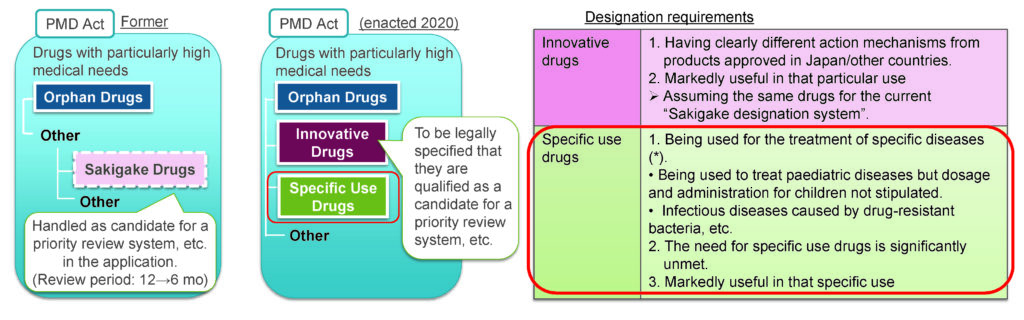
- The Specific Use Drug Designation System, or SAKIGAKE, accelerates the approval process for drugs that address unmet medical needs, including those for pediatric patients, by providing expedited review and approval for drugs that have shown promising results in early-phase clinical trials.
The pediatric drug development environment in Japan
Japanese pharmaceutical companies have been reluctant to embrace pediatric drug development due to:
- Ethical concerns about conducting clinical trials in children
- Limited market size
- The high cost of pediatric drug development
- The rarity of many pediatric diseases
To ensure safety and efficacy, pediatric dose finding studies are required for all new drug and biologic programs seeking approval for use in pediatric patients. However, pharmaceutical companies face challenges, including difficulty in recruiting pediatric patients for clinical trials and determining the appropriate dosage and dosing regimen for pediatric patients.
International regulatory collaboration
The US Food and Drug Administration (FDA) Pediatric Study Plan (PSP) and the EU PIP (Pediatric Investigation Plan) requirements have raised awareness in Japanese pharmaceutical companies of the importance of conducting pediatric studies.
A pediatric cluster was initiated between the PMDA and other regulatory agencies with the aim of promoting the development and approval of safe and effective drugs for use in pediatric populations.
The growing importance of quantitative modeling and simulation in Japan
Quantitative modeling and simulation (M&S) have gained attention in drug development in recent years. Regarding the utilization of M&S in NDA documents for new molecular entities (NMEs) submitted to the PMDA in 2020, reports on population pharmacokinetic (PPK) and population pharmacokinetic/pharmacodynamic (PPK/PD) or exposure-response (E-R) analyses were submitted for ~70% (29/43) and 50% (22/43), respectively.
In addition, when limited to small molecules, the percentage of physiologically-based pharmacokinetic (PBPK) modeling included in NDA documents for NMEs from Japan fiscal year (JFY) 2019 to JFY 2020 was ~40% (9/25 in JFY 2019 and 9/23 in JFY 2020) (learn more about the benefits of using PBPK). Therefore, M&S approaches such as PPK, PPK-PD, E-R and PBPK modeling are becoming more commonly used in Japan. The PMDA launched the M&S project team (M&SPT) to address the increasing number of M&S submissions in recent years. In addition, the PMDA has published guidelines for M&S, which serve as a communication tool between pharmaceutical companies and the agency.
The M&SPT plays a pivotal role in addressing the utilization of these relatively new techniques and emphasizes the importance of promoting M&S in drug development in Japan.
The future of pediatric drug development in Japan and the APAC region
There are several initiatives and trends that are expected to drive progress in this area:
- Increasing emphasis on rare and neglected diseases (learn more about rare disease drug development here)
- Greater international collaboration
- Growing importance of patient-centric drug development
结论
The PMDA’s efforts to improve the regulatory framework for developing safe and effective medications for children are commendable. To advance pediatric drug development in Japan and the APAC region, there is a need to strengthen collaboration between industry, government, and academia.
Certara offers regulatory support services for pharmaceutical companies in Japan and other APAC countries, including pediatric drug development. Our experts assist with study designs and protocols, conduct pharmacokinetic modeling and simulation studies, and offer support for optimizing interactions with regulatory authorities.
To learn more about how Certara is accelerating pediatric drug development, visit: https://www.certara.com/services/practice-areas/pediatric-practice/
参考文献
- Sakiyama M. Promotion of Pediatric Drug Development by Industry, Government and Academia – What Has Changed, What Has Been Done and What Is Necessary for the Further Progress? From the perspective of the PMDA. Presented at DIA. 2019. Available at: https://www.pmda.go.jp/files/000233569.pdf
- Sakiyama M. Paediatric Drug Development in Japan and International Regulatory Collaboration. Presented at the European Forum for Good Clinical Practice. Available at: https://www.pmda.go.jp/files/000244626.pdf.
- Pharmaceuticals and Medical Devices Agency. PMDA website. Available from: https://www.pmda.go.jp/english/
- FDA. Noninferiority clinical trials to establish effectiveness: guidance for industry. Silver Spring, MD: US Food and Drug Administration; 2016 Available from: https://www.fda.gov/media/134069/download.
- Certara. Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) Renews Licenses of Certara’s Software for Evaluating Regulatory Submissions. Certara; 2019. Available from: https://www.certara.com/pressrelease/japans-pharmaceuticals-and-medical-devices-agency-pmda-renews-licenses-of-certaras-software-for-evaluating-regulatory-submissions/
- Tsubouchi H, Hisaka A, Yamada A, et al. Activity and perspective on quantitative modeling and simulation in Japan: Update from the Pharmaceuticals and Medical Devices Agency. CPT Pharmacometrics Syst Pharmacol. 2020;9(12):679-681. doi: 10.1002/psp4.12868. Available from: https://ascpt.onlinelibrary.wiley.com/doi/epdf/10.1002/psp4.12868
- Kijima S, Yoshida S, Ochiai Y. Activity and perspective on quantitative modeling and simulation in Japan: Update from the Pharmaceuticals and Medical Devices Agency. CPT Pharmacometrics Syst Pharmacol. 2022 Dec;11(12):1552-1555. https://ascpt.onlinelibrary.wiley.com/doi/epdf/10.1002/psp4.12868




