2024 年 12 月 5 日

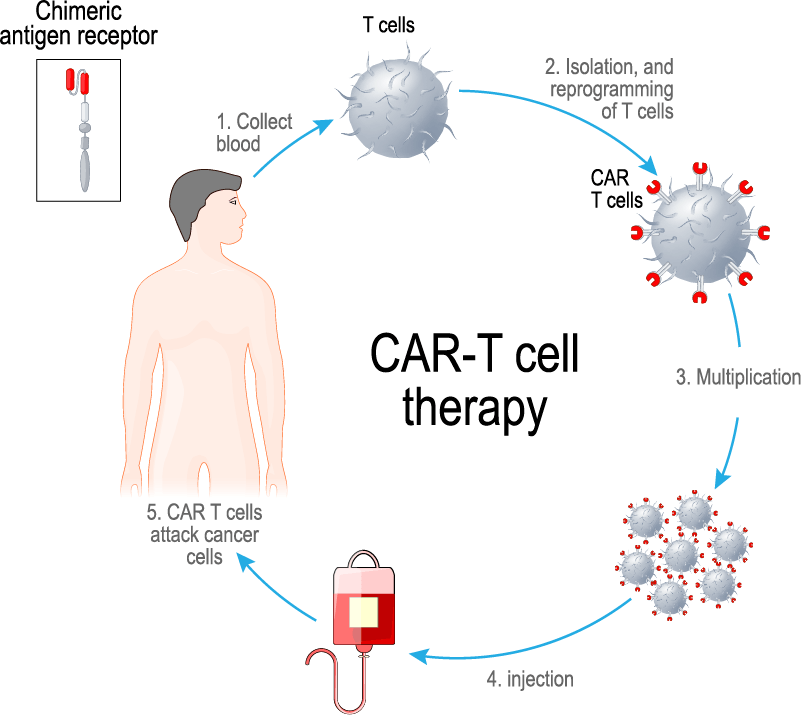
Using Clinical Pharmacology & Pharmacometrics to Accelerate Cell Therapy Development: A Patient’s CAR-T Journey
To learn more about best practices for cell therapeutics development, please watch this webinar.
参考文献
Ogasawara K, Dodds M, et al. Population Cellular Kinetics of Lisocabtagene Maraleucel, an Autologous CD19‑Directed Chimeric Antigen Receptor T‑Cell Product, in Patients with Relapsed/Refractory Large B‑Cell Lymphoma. Clinical Pharmacokinetics. 2021 Dec;60(12):1621-1633. doi: 10.1007/s40262-021-01039-5. Epub 2021 Jun 14.
FDA draft guidance, March 2022: Considerations for the development of CAR-T cell products
This blog was originally published on 2022 年 9 月 9 日 and has since been updated.

Senior Distinguished Scientist
Rajesh is a scientific key opinion leader with 25+ years in drug development, specializing in model-informed strategies for biologics, vaccines, and small molecules. Currently a Senior Distinguished Scientist at Certara, he leads strategic consulting and the CDDS centers of excellence. Previously, he founded Merck’s quantitative clinical pharmacology department and held key roles at Aventis and Bristol-Myers Squibb. Rajesh holds a PhD in Pharmaceutical Sciences (University of British Columbia) and an MBA in Strategy and Innovation (Warwick). Consistently recognized among the top 2% of influential scientists, his work includes 100+ publications, 89 posters, and 4 books. He is an elected fellow of AAPS.

副总裁
Kathryn 是 Certara 综合药物开发部的临床药理学顾问和团队领导。此外,她还领导复杂生物制剂综合业务领域,专门为客户提供细胞疗法、RNA 技术、基因疗法、融合蛋白、ADC、双特异性抗体等领域的专家支持。在2020年加入Certara之前,Kathryn 在制药行业的临床药理学和药物计量学领域工作了超过20年,工作涉及各个研发阶段。
Contact us heading