Your Guide to Modernizing Non-Compartmental Analysis (NCA) Workflows
Why NCA Needs a Modern Approach
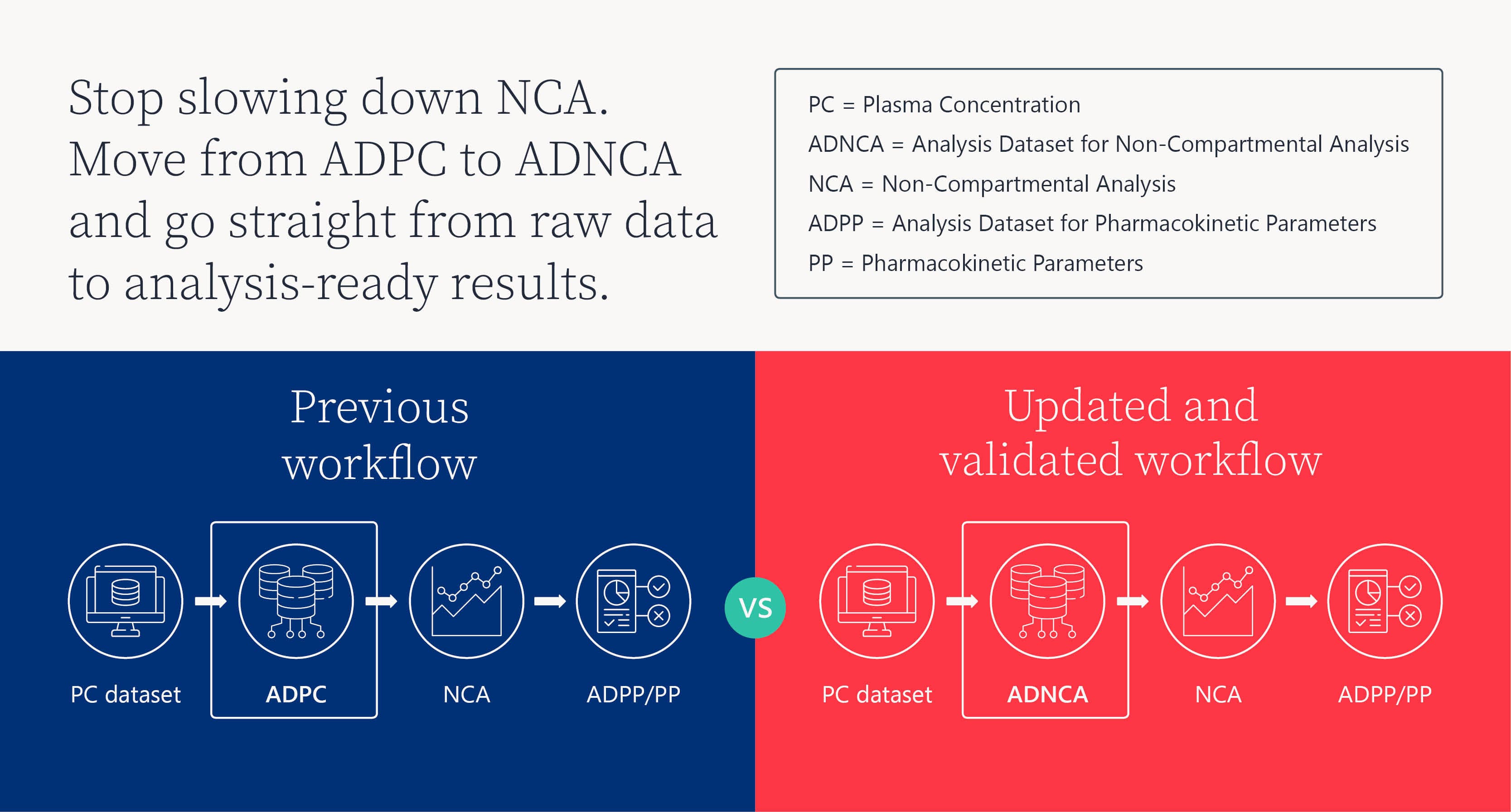
When regulatory deadlines are tight, delays in pharmacokinetic (PK) data preparation can derail timelines and shorten valuable patent life. Traditional NCA workflows, once considered fast and flexible, are now slowed by manual formatting, inconsistent variable handling, and rework that generates avoidable queries and stretches submission timelines.
The Analysis Dataset Model for Non-Compartmental Analysis (ADNCA) is a formal CDISC ADaM data standard that streamlines workflows, strengthens compliance, and ensures submission-ready PK datasets.
In this guide, you’ll discover the Top 10 Things You Need to Know About ADNCA datasets including expert tips from leading PK scientists so you can modernize workflows, accelerate submissions, and build regulatory confidence.
What You’ll Learn
✔ Build regulatory confidence by aligning with FDA, PMDA, and CDISC expectations.
✔ Future-proof submissions as ADNCA adoption grows among global regulators.
✔ Standardize workflows with 50+ validated NCA-related variables.
✔ Integrate seamlessly with Phoenix WinNonlin™️ for interim and final analyses.
✔ Automate reproducibly with variables like MRRLT and TRTRINT.
✔ Collaborate effectively with CROs and partners through harmonized datasets.
✔ Simplify validation in Pinnacle 21 Enterprise.
✔ Reduce reviewer burden with faster replication and fewer information requests.
Case Study Spotlight: ADNCA in Action
One sponsor adopted ADNCA mid-development, aligning datasets across three ongoing studies. The impact was immediate:
- 40% reduction in dataset preparation time
- Interim analysis turnaround cut from 10 days to 3
- Avoided a major IR by delivering data in a familiar, review-ready format
Linking PK Parameters to Clinical Outcomes
ADNCA’s value becomes strongest when paired with solid pharmacokinetic principles:
- In many therapeutic areas, higher steady-state concentrations correlate with improved treatment responses, making consistent exposure a key driver of effectiveness
- For some therapies, trough-based monitoring is less reliable. Exposure-guided dosing using 24-hour exposure ranges is increasingly adopted to balance efficacy and patient safety
ADNCA supports clarity, reproducibility and confidence in PK data analysis.
Implementation Tips
- Start early: build ADNCA mapping specs during study setup
- Leverage Phoenix templates: automate tables, figures and listings (TFL)
- Maintain a metadata repositor: keep specifications consistent across studies
- Validate continuously: use Pinnacle 21 Enterprise pre- and post-database lock
Streamline Your NCA Workflows
ADNCA is redefining NCA. Simplify workflows, strengthen compliance, and accelerate submissions. Download the guide!