2025 年 1 月 10 日

罕见病药物开发的挑战与机遇
Learn more about the opportunities and challenges in rare disease drug development in our white paper.
参考文献
FDA Summary Basis of Approval. URL: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/216718Orig1s000SumR.pdf
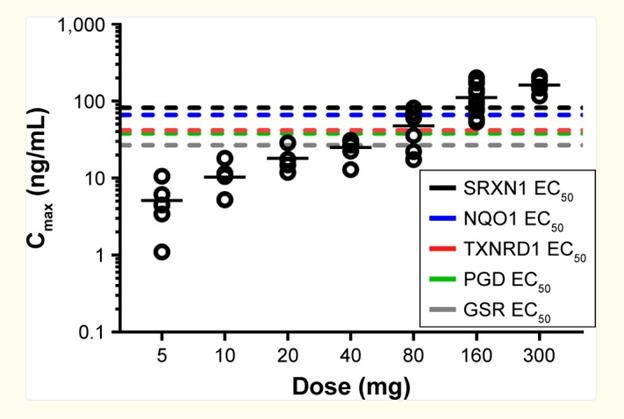
Reisman S et al. Pharmacokinetics and pharmacodynamics of the novel Nrf2 activator omaveloxolone in primates. Drug Des Devel Ther 2019 Apr 17;13:1259–1270.
Almond L et al. Prediction of Drug-Drug Interactions Arising from CYP3A induction Using a Physiologically Based Dynamic Model. Drug Metab Dispos 44:821–832, June 2016.

Senior Distinguished Scientist
Rajesh is a scientific key opinion leader with 25+ years in drug development, specializing in model-informed strategies for biologics, vaccines, and small molecules. Currently a Senior Distinguished Scientist at Certara, he leads strategic consulting and the CDDS centers of excellence. Previously, he founded Merck’s quantitative clinical pharmacology department and held key roles at Aventis and Bristol-Myers Squibb. Rajesh holds a PhD in Pharmaceutical Sciences (University of British Columbia) and an MBA in Strategy and Innovation (Warwick). Consistently recognized among the top 2% of influential scientists, his work includes 100+ publications, 89 posters, and 4 books. He is an elected fellow of AAPS.
Contact us heading