2025 年 9 月 5 日
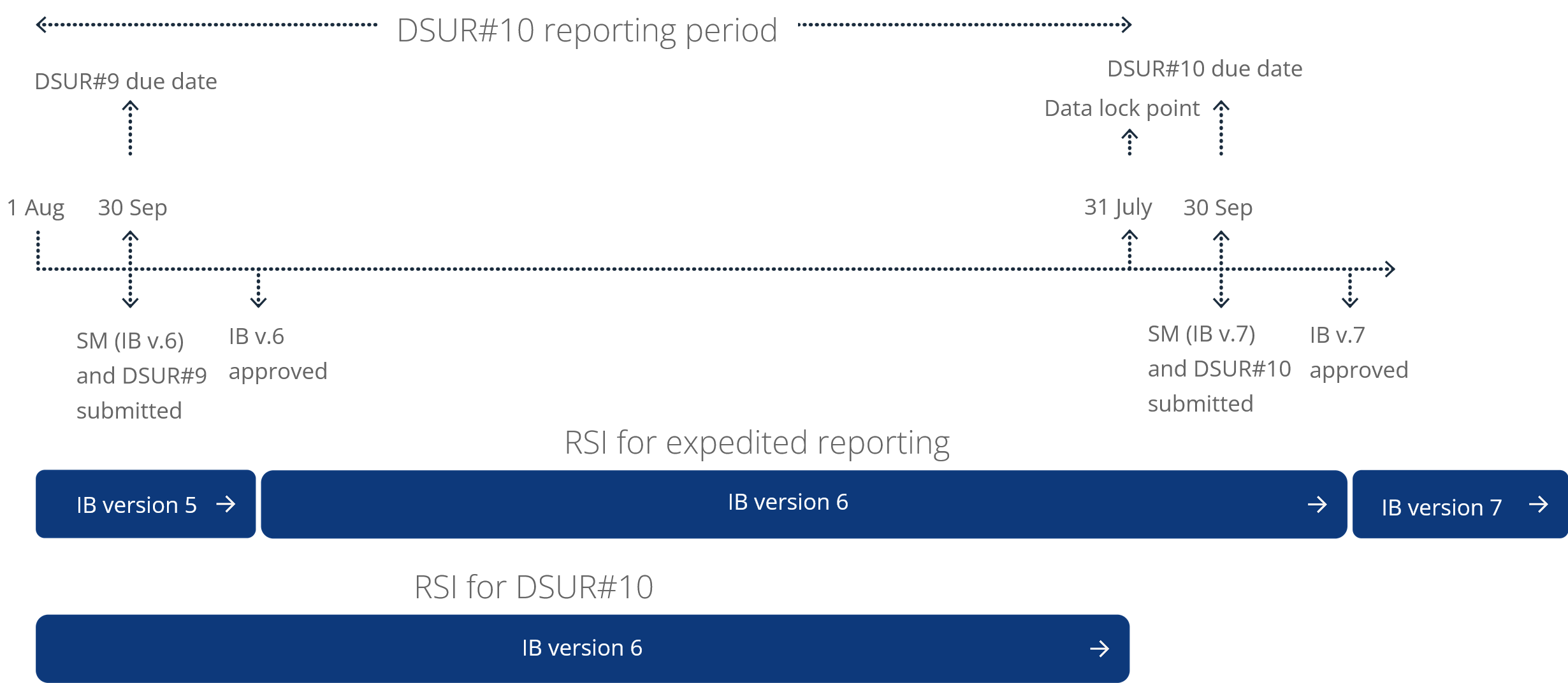
Figure 1: Example of the IB RSI update following the DSUR reporting period; adapted from CTR (EU) No. 536/2014 Q&A guidance document 2
Abbreviations: DSUR, Development safety update report; CTR, Clinical Trial Regulation; EU, European Union; IB, Investigator’s brochure; RSI, Reference Safety Information; SM, Substantial modification.

Adhering to regional guidelines to meet strict regulatory deadlines can be challenging. Certara regulatory writers are experts in authoring pharmacovigilance documents, and harnessing their expertise is a great asset to any biopharmaceutical company. For more information on pharmacovigilance documents, read this white paper.
参考文献
- Clinical Trials Regulation (Regulation (EU) No. 536/2014). Available from: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/clinical-trials-human-medicines/clinical-trials-regulation.
- The rules governing medicinal products in the European Union volume 10 – Guidance documents applying to clinical trials. Clinical Trials Regulation (EU) NO 536/2014. Questions and Answers. Version 7.1. Accessed on 29 April 2025. Available from: https://health.ec.europa.eu/latest-updates/questions-and-answers-document-regulation-eu-5362014-version-5-january-2022-2022-02-01_en.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline: Development safety update report E2F. Accessed on 29 April 2025. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-e2f-development-safety-update-report-step-5_en.pdf.
- Clinical Trial Facilitation Group (CTFG) Q&A document – Reference safety information Accessed on 29 April 2025. Available from: https://www.hma.eu/fileadmin/dateien/Human_Medicines/01-About_HMA/Working_Groups/CTFG/2017_11_CTFG_Question_and_Answer_on_Reference_Safety_Information_2017.pdf.
- Guideline on how to increase transparency when presenting safety information in the Development Safety Update Report (DSUR): region-specific requirements for Canada and the United Kingdom. Last accessed 29 April 2025. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/993808/DSUR-Guideline-08June2021.pdf.

Principal Regulatory Writer
Dr. Nicholas Churton is a principal regulatory writer with over 10 years of experience working in a clinical research organization. He has authored multiple regulatory documents across various therapeutic areas and multiple phases, specializing in aggregate safety reporting documents such as development safety update reports (DSURs), periodic benefit-risk evaluation reports (PBRERs), risk management plans (RMPs), periodic adverse drug experience reports (PADERs), and responses to health authority queries. He has experience in managing large cross-functional teams on pivotal projects in addition to developing training resources and providing oversight of junior writers.
常见问题解答
What is the EU Clinical Trials Regulation (CTR) 536/2014, and how does it impact my annual safety reports?
The EU CTR No. 536/2014, which replaced the Clinical Trials Directive in January 2022, harmonizes clinical trial processes across the European Union (EU). It introduces additional requirements for annual safety reports (ASRs), also known as development safety update reports (DSURs), including updated Reference Safety Information (RSI) alignment, enhanced regional requirements, and modified data presentation standards.
How should I handle RSI updates under the new regulation?
Under EU CTR No. 536/2014, the RSI section of your Investigator’s brochure should be updated annually to align with your DSUR data lock point and submitted within one month of the DSUR. This updated RSI becomes effective for the subsequent reporting period, ensuring consistency in expectedness assessments for serious adverse reactions.
What new regional requirements must be included in EU/EEA DSUR submissions?
The CTR Q&A guidance document requires five key regional elements: a cumulative summary tabulation of serious adverse reactions (SARs), lists of subjects who died during the reporting period, lists of subjects who withdrew due to adverse events, a comprehensive safety signal review with tabulated outcomes, and inclusion of EU clinical trial numbers alongside protocol codes.
How has subject privacy protection changed under the new regulation?
To comply with Article 43.3 of the CTR and protect patient rights, you must remove all subject identifiers from data line listings. This includes using only case identification numbers and Study IDs in SAR listings, while ensuring that death and withdrawal listings cannot identify natural persons.
联系我们