With nine FDA-approved drugs today, 100+ in clinical development and several hundred in the preclinical stage, the market for bispecific antibodies is projected to grow to >$30B by 2028. The distinct advantages of bispecific antibodies, such as improved selectivity and specificity, increased efficacy, and lower toxicity, will result in measurable benefits for cancer patients. But bispecific antibodies are not the same as combination therapy and require a greater understanding of the drug (s) and biological system being targeted.
This work requires the use of a mechanistic modeling approach, specifically Quantitative Systems Pharmacology (QSP), which benefit drug developers in 4 ways:
- Selection of starting dose: getting to IND approval faster and at lower cost.
- Defining efficacious dose: sources of variability and mechanism of action of two antibodies is not additive, so traditional dose escalation methods often fail.
- Managing safety concerns: reduce the impact of cytokine release syndrome (CRS) via modeling.
- Predicting and managing between patient variability: using virtual twins to amplify patient characteristics and differentiation to employ the best therapeutic approach.
Bispecific Antibodies: Advantages and Opportunities for Revolutionizing the Field of Immuno-oncology
As opposed to a monoclonal antibody, in which both halves” of the Y-shaped molecule are identical and bind to the same antigen target, the two halves of the bispecific antibody are different, allowing simultaneous binding to two different targets. These may be antigens on two different cells, or two different antigens on the same cell. By targeting two antigens or epitopes, bispecific antibodies can deliver multiple physiological and anti-tumor responses. As opposed to combination therapy, the two mAbs may work independently or in connection with one another, but patients only need to take a single treatment. The synergistic features of the bispecific antibodies enable a wider range of therapeutic applications and are propelled by the growth in cancer patients and types, increased demand for targeted therapies, improved efficacy and safety profiles, and desire for personalized medicine.
Already in development, the next generation of targeted therapies are multispecifics, protein-based therapeutic molecules that can bind to more than two targets simultaneously.
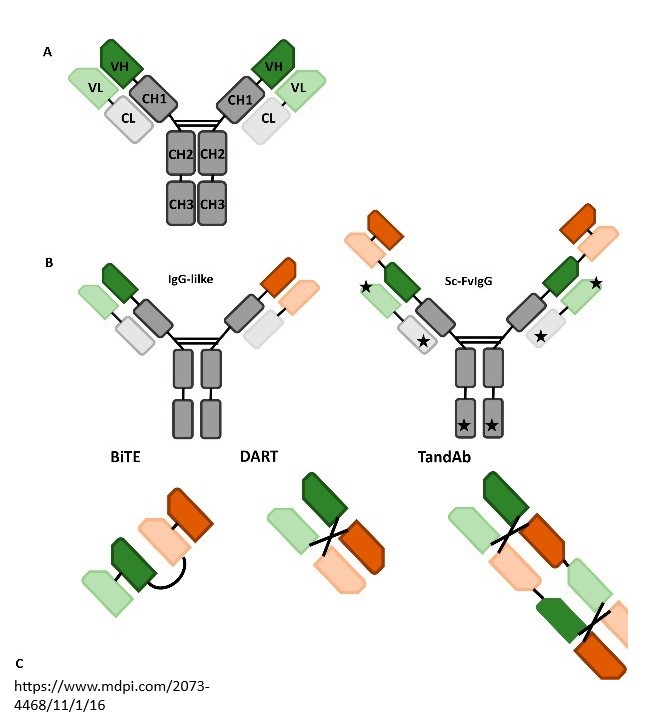
The Challenges in Bispecific Antibody Development
While of great therapeutic potential, bispecific antibodies (and multispecifics) are complex modalities with drug development challenges to overcome including:
- Selection of optimal targets and modalities appropriate for the mechanism of action.
- Design of bispecific antibodies including optimal affinities for each target.
- Mechanistic complexities such as role of avidity and the bell-shaped concentration response relationship.
- Selection of relevant starting doses using a minimal anticipated biological effect level.
- Predicting efficacious dose despite nonintuitive dose response relationships.
- Navigating efficacy vs. toxicity.
A unique issue for bispecific antibodies relates to dose prediction, as per the seminal tutorial from Betts and van der Graaf1. “Historically, in oncology drug development, efficacy has been assumed to be dose related and cancer drugs are escalated to the maximum tolerated dose.”The complex mechanism of action of bispecific antibodies can result in nonlinear relationships between dose and trimer exposure, resulting in a bell- shaped concentration vs response curve as shown in the diagram.
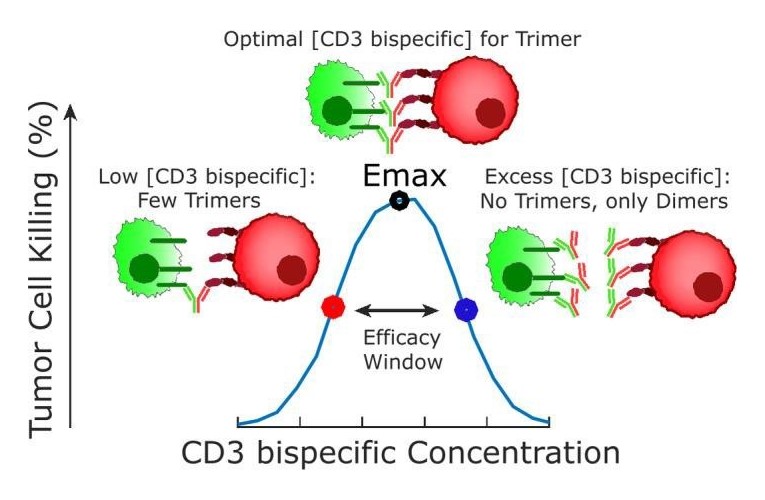
Mechanistic, QSP modeling approaches are a powerful integrative tool to understand the complexities and aid in bispecific antibody design and optimization, clinical translation, trial design, and prediction of regimens and strategies to reduce dose limiting toxicities of bispecific antibodies. QSP modeling is increasingly used to support regulatory (IND) submissions.
Case Studies Affirm Value of QSP in Translational Stages of Bispecific and Multispecific Development
- Optimizing therapeutic index: For one sponsor, we built computational models of tissue targeting bispecifics to facilitate drug design and minimize risk in patients. Effectively, this provided useful information to optimize therapeutic window.
- Selection of starting dose: The sponsor had two bispecific molecules moving toward IND submittal in a six-month period. The first followed the traditional MABEL approach, the second replaced FIH prediction using a QSP model. The QSP model enabled the sponsor to save two full dose levels, attaining the IND faster and at lower cost. For our partner, Genmab, we developed a semi-mechanistic PK/PD model that predicted trimer levels and PD-L1 RO in tumors, and this supported dose selection and Phase 1/2 trial expansion for GEN1046, their investigational, first-in-class bispecific antibody.
- Defining efficacious dose: In this example for a T cell engager bispecific antibody, the QSP model was able to accurately predict the observed clinical efficacy dose range when the dose was estimated based on in vivo-data derived nACT (TCE and tumor cell normalized to number of tumor cells (nACT).
- Managing safety concerns: A common side effect of bispecific antibody immunotherapy is cytokine release syndrome (CRS), a potentially life-threatening complication in which activated immune cells release a large number of cytokines into the bloodstream, resulting in systemic inflammation. QSP modeling can be used to predict CRS. In this case example, the QSP model was used to assess the translatability of preclinical data and predicted that the molecule could achieve efficacy without compromising cytotoxic activity on human tumors to cross-reactivity considerations.
- Predicting and managing between patient variability: Certara’s Virtual Twin technology creates a computer-simulated model of each patient, replicating the patient’s various attributes that affect a drug’s fate in their body and hence its effects. This mechanistic modeling approach can predict the optimal drug dosing regimen for an individual patient – one that maximizes therapeutic benefit while minimizing side effects – by evaluating the impact of different drug doses, schedules, and combinations in the patients’ in silico ‘virtual twin’ first.
Certara Simcyp has developed a platform for mechanistic modeling of bispecific antibodies using our QSP Designer software, which was introduced via this poster paper at the September 2023 Annual Consortium meeting. With >20 completed bispecific and multispecific projects and a PBPK/QSP platform, we can perform a FIH dose prediction project in less than two months.
Betts, A and van der Graaf, P., “Mechanistic Quantitative Pharmacology Strategies for the Early Clinical Development of Bispecific Antibodies in Oncology,” Clin Pharmacol Ther. 2020 Sep;108(3):528-541.
This blog was originally posted on 2023 年 10 月 12 日 and updated on 2024 年 9 月 12 日.




